Systematic Name For Cof2 S
 | |
 | |
| Names | |
|---|---|
| IUPAC proper noun Cobalt(II) fluoride | |
| Other names cobalt difluoride | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.030.044 |
| EC Number |
|
| PubChem CID |
|
| RTECS number |
|
| UNII |
|
| CompTox Dashboard (EPA) |
|
| InChI
| |
| SMILES
| |
| Backdrop | |
| Chemical formula | CoFii |
| Molar mass | 96.93 g/mol |
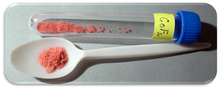
| Appearance | Cherry-red crystalline solid |
| Density | 4.46 g/cmiii (anhydrous) 2.22 m/cm3 (tetrahydrate) |
| Melting point | 1,217 °C (2,223 °F; 1,490 Thousand) |
| Humid point | one,400 °C (2,550 °F; 1,670 K) |
| Solubility in water | 1.4 g/100 mL (25 °C) |
| Solubility | soluble in HF insoluble in alcohol, ether, benzene |
| Magnetic susceptibility (χ) | +9490.0·x−vi cmiii/mol |
| Structure | |
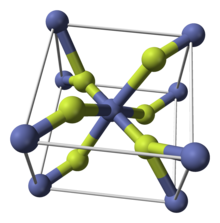
| Crystal construction | tetragonal (a,hydrous) orthorhombic (tetrahydrate) |
| Hazards | |
| NFPA 704 (burn down diamond) | 3 0 0 |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | oral (rat): 150 mg/kg |
| Related compounds | |
| Other anions | cobalt(II) oxide, cobalt(II) chloride |
| Other cations | atomic number 26(Ii) fluoride, nickel(II) fluoride |
| Related compounds | cobalt trifluoride |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |

Anhydrous cobalt(II) fluoride
Cobalt(Ii) fluoride is a chemical compound with the formula (CoF2). It is a pink crystalline solid chemical compound[1] [2] which is antiferromagnetic at low temperatures (TN=37.7 K)[3] The formula is given for both the red tetragonal crystal, (CoF2), and the tetrahydrate ruby orthogonal crystal, (CoFii·4HtwoO). CoF2 is used in oxygen-sensitive fields, namely metallic production. In low concentrations, it has public health uses. CoF2 is sparingly soluble in water. The compound can exist dissolved in warm mineral acid, and volition decompose in boiling water. Yet the hydrate is water-soluble, especially the di-hydrate CoFtwo·2H2 O and tri-hydrate CoF2·3HtwoO forms of the compound. The hydrate volition also decompose with estrus.
Similar some other metal difluorides, CoF2 crystallizes in the rutile structure, which features octahedral Co centers and planar fluorides.[4]
Preparation [edit]
Cobalt(II) fluoride can be prepared from anhydrous cobalt(II) chloride or cobalt(Ii) oxide in a stream of hydrogen fluoride:
- CoCl2 + 2HF → CoF2 + 2HCl
- CoO + 2HF → CoFtwo + HiiO
It is produced in the reaction of cobalt (III) fluoride with h2o.
The tetrahydrate cobalt(Ii) fluoride is formed by dissolving cobalt(2) in hydrofluoric acid. The anhydrous fluoride tin can be extracted from this past dehydration. Other synthesis can occur at college temperatures. It has been shown that at 500 °C fluorine will combine with cobalt producing a mixture of CoF2 and CoFiii.[5]
Uses [edit]
Cobalt(Ii) fluoride can be used every bit a catalyst to alloy metals. It is besides used for optical deposition, of which it tremendously improves optical quality. Cobalt(II) fluoride is available in most volumes in an ultra high purity limerick. Loftier purity compositions ameliorate optical qualities and its usefulness as a standard.
Assay [edit]
To clarify this compound, Cobalt (2) fluoride can exist dissolved in nitric acid. The solution is and then diluted with water until appropriate concentration for AA or ICP spectrophotometry for the cobalt. A minor amount of salt can be dissolved in cold water and analyzed for fluoride ion by a fluoride ion-selective electrode or ion chromatography.
Chemical Backdrop [edit]
CoF2 is a weak Lewis acid. Cobalt(Ii) complexes are ordinarily octahedral or tetrahedral. As a nineteen-electron species information technology is a good reducing agent, adequately oxidizable into an 18-electron chemical compound. Cobalt(Two) fluoride can be reduced by hydrogen at a 300 °C.
References [edit]
- ^ Pradyot Patnaik (2002), Handbook of Inorganic Chemicals, McGraw-Hill Professional person, ISBN978-0-07-049439-8
- ^ Pashkevich, D. Due south.; Radchenko Due south. Grand.; Mukhortov, D. A., "Article title Estrus Exchange between Cobalt(II) Fluoride Powder and the Wall of Rotating Cylinder" (PDF), Russian Journal of Practical Chemistry, Consultants Bureau, ISSN 1070-4272, archived from the original (PDF) on 2004-09-29, retrieved 2007-03-07
- ^ Ashcroft/Mermin: Solid Land Physics (Tab. 33.two)
- ^ Stout, J. Westward.; Reed, Stanley A. (1954). "The Crystal Structure of MnFii, FeFii, CoF2, NiFii and ZnF2". J. Am. Chem. Soc. 76 (21): 5279–5281. doi:10.1021/ja01650a005.
- ^ J.C. Bailar (1973), Comprehensive Inorganic Chemical science, Pergoamon
External links [edit]
- National Pollutant Inventory - Cobalt fact sheet
- National Pollutant Inventory - Fluoride and compounds fact canvass
- [ane]
Systematic Name For Cof2 S,
Source: https://en.wikipedia.org/wiki/Cobalt%28II%29_fluoride
Posted by: haltertrachattee1941.blogspot.com



0 Response to "Systematic Name For Cof2 S"
Post a Comment