Is Sodium Extracellular Or Intracellular
Learning Objectives
By the finish of this section, you will be able to:
- Explicate the importance of water in the trunk
- Contrast the composition of the intracellular fluid with that of the extracellular fluid
- Explicate the importance of protein channels in the movement of solutes
- Identify the causes and symptoms of edema
The chemical reactions of life have place in aqueous solutions. The dissolved substances in a solution are called solutes. In the human being body, solutes vary in different parts of the torso, but may include proteins—including those that transport lipids, carbohydrates, and, very chiefly, electrolytes. Often in medicine, a mineral dissociated from a salt that carries an electrical charge (an ion) is called and electrolyte. For instance, sodium ions (Na+) and chloride ions (Cl–) are often referred to as electrolytes.
In the body, water moves through semi-permeable membranes of cells and from i compartment of the body to another past a procedure called osmosis. Osmosis is basically the diffusion of water from regions of college concentration to regions of lower concentration, forth an osmotic gradient across a semi-permeable membrane. As a result, water will move into and out of cells and tissues, depending on the relative concentrations of the h2o and solutes institute there. An advisable rest of solutes inside and outside of cells must exist maintained to ensure normal function.
Body H2o Content

Figure i. Water content varies in dissimilar trunk organs and tissues, from every bit little as 8 percent in the teeth to as much as 85 percent in the encephalon.
Man beings are mostly water, ranging from nearly 75 per centum of body mass in infants to about 50–60 pct in developed men and women, to as depression as 45 percentage in one-time historic period. The percentage of trunk water changes with development, considering the proportions of the trunk given over to each organ and to muscles, fat, bone, and other tissues change from infancy to adulthood. Your brain and kidneys have the highest proportions of water, which composes eighty–85 percent of their masses. In contrast, teeth have the lowest proportion of h2o, at viii–ten pct.
Fluid Compartments

Figure ii. The intracellular fluid (ICF) is the fluid inside cells. The interstitial fluid (IF) is office of the extracellular fluid (ECF) between the cells. Blood plasma is the 2d part of the ECF. Materials travel between cells and the plasma in capillaries through the IF.
Body fluids tin exist discussed in terms of their specific fluid compartment, a location that is largely separate from another compartment by some form of a physical barrier. The intracellular fluid (ICF) compartment is the system that includes all fluid enclosed in cells by their plasma membranes. Extracellular fluid (ECF) surrounds all cells in the trunk. Extracellular fluid has two primary constituents: the fluid component of the blood (called plasma) and the interstitial fluid (IF) that surrounds all cells non in the blood.
Intracellular Fluid
The ICF lies within cells and is the principal component of the cytosol/cytoplasm. The ICF makes up about 60 percentage of the total h2o in the human body, and in an average-size adult male, the ICF accounts for about 25 liters (7 gallons) of fluid. This fluid volume tends to be very stable, because the amount of h2o in living cells is closely regulated. If the amount of water within a cell falls to a value that is besides low, the cytosol becomes too full-bodied with solutes to conduct on normal cellular activities; if too much water enters a cell, the prison cell may burst and be destroyed.

Figure 3. Most of the water in the body is intracellular fluid. The second largest volume is the interstitial fluid, which surrounds cells that are not claret cells.
Extracellular Fluid
The ECF accounts for the other one-3rd of the trunk's h2o content. Approximately 20 percent of the ECF is institute in plasma. Plasma travels through the body in blood vessels and transports a range of materials, including blood cells, proteins (including clotting factors and antibodies), electrolytes, nutrients, gases, and wastes. Gases, nutrients, and waste materials travel between capillaries and cells through the IF. Cells are separated from the IF past a selectively permeable cell membrane that helps regulate the passage of materials between the IF and the interior of the cell.
The body has other h2o-based ECF. These include the cerebrospinal fluid that bathes the brain and spinal cord, lymph, the synovial fluid in joints, the pleural fluid in the pleural cavities, the pericardial fluid in the cardiac sac, the peritoneal fluid in the peritoneal cavity, and the aqueous humor of the eye. Because these fluids are outside of cells, these fluids are also considered components of the ECF compartment.
Limerick of Body Fluids
The compositions of the ii components of the ECF—plasma and IF—are more similar to each other than either is to the ICF. Blood plasma has loftier concentrations of sodium, chloride, bicarbonate, and protein. The IF has high concentrations of sodium, chloride, and bicarbonate, but a relatively lower concentration of protein. In contrast, the ICF has elevated amounts of potassium, phosphate, magnesium, and protein. Overall, the ICF contains high concentrations of potassium and phosphate ( [latex]{\text{HPO}}_{4}^{2-}[/latex] ), whereas both plasma and the ECF contain high concentrations of sodium and chloride.

Figure 4. The graph shows the composition of the ICF, IF, and plasma. The compositions of plasma and IF are similar to ane another merely are quite different from the limerick of the ICF.
Practice Question
Watch this video to learn more about body fluids, fluid compartments, and electrolytes. When blood volume decreases due to sweating, from what source is water taken in by the blood?
Show Answer
The interstitial fluid (IF).
Near body fluids are neutral in charge. Thus, cations, or positively charged ions, and anions, or negatively charged ions, are balanced in fluids. As seen in the previous graph, sodium (Na+) ions and chloride (Cl–) ions are concentrated in the ECF of the torso, whereas potassium (K+) ions are concentrated within cells. Although sodium and potassium tin can "leak" through "pores" into and out of cells, respectively, the loftier levels of potassium and low levels of sodium in the ICF are maintained by sodium-potassium pumps in the cell membranes. These pumps use the energy supplied past ATP to pump sodium out of the cell and potassium into the cell.

Effigy 5. The sodium-potassium pump is powered by ATP to transfer sodium out of the cytoplasm and into the ECF. The pump besides transfers potassium out of the ECF and into the cytoplasm. (credit: modification of piece of work by Mariana Ruiz Villarreal)
Fluid Movement between Compartments
Hydrostatic pressure, the strength exerted by a fluid against a wall, causes motility of fluid between compartments. The hydrostatic pressure level of claret is the force per unit area exerted by blood against the walls of the blood vessels by the pumping activeness of the heart. In capillaries, hydrostatic pressure level (besides known as capillary claret pressure) is college than the opposing "colloid osmotic pressure" in blood—a "abiding" pressure primarily produced by circulating albumin—at the arteriolar end of the capillary. This pressure forces plasma and nutrients out of the capillaries and into surrounding tissues. Fluid and the cellular wastes in the tissues enter the capillaries at the venule finish, where the hydrostatic pressure is less than the osmotic pressure in the vessel. Filtration pressure level squeezes fluid from the plasma in the blood to the IF surrounding the tissue cells. The surplus fluid in the interstitial infinite that is non returned straight dorsum to the capillaries is tuckered from tissues by the lymphatic system, and then re-enters the vascular system at the subclavian veins.

Figure vi. Net filtration occurs near the arterial end of the capillary since capillary hydrostatic pressure level (CHP) is greater than blood colloidal osmotic pressure (BCOP). There is no cyberspace movement of fluid near the midpoint of the capillary since CHP = BCOP. Net reabsorption occurs near the venous stop of the capillary since BCOPis greater than CHP.
Practice Question
Watch this video to come across an explanation of the dynamics of fluid in the body'southward compartments. What happens in the tissue when capillary blood pressure is less than osmotic force per unit area?
Show Answer
Fluid enters the capillaries from interstitial spaces.
Hydrostatic pressure is especially important in governing the motion of h2o in the nephrons of the kidneys to ensure proper filtering of the blood to form urine. As hydrostatic pressure in the kidneys increases, the amount of h2o leaving the capillaries also increases, and more urine filtrate is formed. If hydrostatic pressure in the kidneys drops too low, as can happen in dehydration, the functions of the kidneys will be impaired, and less nitrogenous wastes volition be removed from the bloodstream. Extreme dehydration tin can result in kidney failure.
Fluid too moves betwixt compartments forth an osmotic gradient. Recall that an osmotic gradient is produced by the divergence in concentration of all solutes on either side of a semi-permeable membrane. The magnitude of the osmotic gradient is proportional to the difference in the concentration of solutes on one side of the cell membrane to that on the other side. Water will move by osmosis from the side where its concentration is high (and the concentration of solute is low) to the side of the membrane where its concentration is low (and the concentration of solute is high). In the body, water moves past osmosis from plasma to the IF (and the opposite) and from the IF to the ICF (and the reverse). In the torso, h2o moves constantly into and out of fluid compartments every bit conditions change in different parts of the body.
For example, if you are sweating, you lot will lose water through your peel. Sweating depletes your tissues of water and increases the solute concentration in those tissues. Equally this happens, water diffuses from your blood into sweat glands and surrounding skin tissues that have become dehydrated because of the osmotic slope. Additionally, as water leaves the blood, it is replaced by the water in other tissues throughout your body that are not dehydrated. If this continues, dehydration spreads throughout the body. When a dehydrated person drinks h2o and rehydrates, the water is redistributed past the aforementioned slope, but in the opposite direction, replenishing water in all of the tissues.
Solute Motion between Compartments
The movement of some solutes between compartments is active, which consumes energy and is an agile transport process, whereas the move of other solutes is passive, which does not require free energy. Agile transport allows cells to move a specific substance against its concentration gradient through a membrane protein, requiring free energy in the form of ATP. For instance, the sodium-potassium pump employs active send to pump sodium out of cells and potassium into cells, with both substances moving against their concentration gradients.
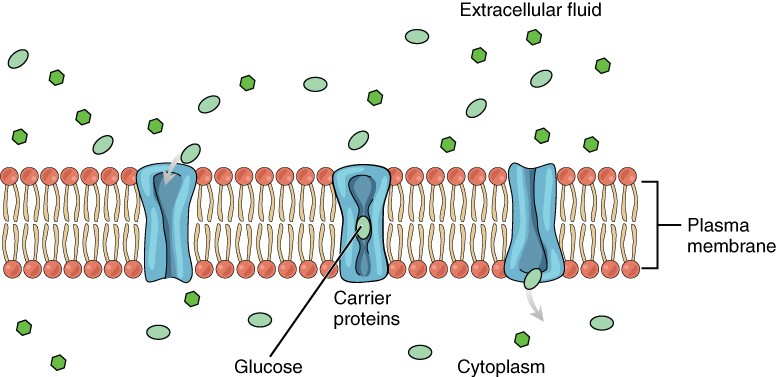
Figure seven. Glucose molecules use facilitated diffusion to move down a concentration gradient through the carrier protein channels in the membrane. (credit: modification of piece of work by Mariana Ruiz Villarreal)
Passive send of a molecule or ion depends on its power to pass through the membrane, as well as the existence of a concentration gradient that allows the molecules to lengthened from an area of college concentration to an surface area of lower concentration. Some molecules, like gases, lipids, and water itself (which also utilizes water channels in the membrane called aquaporins), skid fairly easily through the cell membrane; others, including polar molecules like glucose, amino acids, and ions practice not. Some of these molecules enter and leave cells using facilitated transport, whereby the molecules move down a concentration slope through specific protein channels in the membrane. This process does non require energy. For example, glucose is transferred into cells past glucose transporters that use facilitated transport.
Disorders of the Fluid Balance: Edema
Edema is the accumulation of excess water in the tissues. It is most mutual in the soft tissues of the extremities. The physiological causes of edema include h2o leakage from blood capillaries. Edema is almost always acquired past an underlying medical condition, by the apply of certain therapeutic drugs, by pregnancy, by localized injury, or by an allergic reaction. In the limbs, the symptoms of edema include swelling of the subcutaneous tissues, an increment in the normal size of the limb, and stretched, tight skin. One quick style to check for subcutaneous edema localized in a limb is to press a finger into the suspected area. Edema is likely if the depression persists for several seconds after the finger is removed (which is called "pitting").
Pulmonary edema is excess fluid in the air sacs of the lungs, a common symptom of centre and/or kidney failure. People with pulmonary edema likely will experience difficulty breathing, and they may experience chest pain. Pulmonary edema can exist life threatening, because it compromises gas exchange in the lungs, and anyone having symptoms should immediately seek medical intendance.
In pulmonary edema resulting from middle failure, excessive leakage of water occurs considering fluids get "backed up" in the pulmonary capillaries of the lungs, when the left ventricle of the middle is unable to pump sufficient blood into the systemic circulation. Because the left side of the heart is unable to pump out its normal volume of blood, the claret in the pulmonary apportionment gets "backed upward," starting with the left atrium, and then into the pulmonary veins, and then into pulmonary capillaries. The resulting increased hydrostatic pressure within pulmonary capillaries, as blood is all the same coming in from the pulmonary arteries, causes fluid to be pushed out of them and into lung tissues.
Other causes of edema include damage to blood vessels and/or lymphatic vessels, or a decrease in osmotic force per unit area in chronic and severe liver disease, where the liver is unable to manufacture plasma proteins. A decrease in the normal levels of plasma proteins results in a decrease of colloid osmotic force per unit area (which counterbalances the hydrostatic pressure level) in the capillaries. This process causes loss of water from the claret to the surrounding tissues, resulting in edema.

Figure 8. An allergic reaction can cause capillaries in the hand to leak excess fluid that accumulates in the tissues. (credit: Jane Whitney)
Balmy, transient edema of the feet and legs may be caused by sitting or standing in the same position for long periods of time, as in the work of a toll collector or a supermarket cashier. This is considering deep veins in the lower limbs rely on skeletal muscle contractions to button on the veins and thus "pump" claret back to the center. Otherwise, the venous blood pools in the lower limbs and can leak into surrounding tissues.
Medications that can consequence in edema include vasodilators, calcium channel blockers used to treat hypertension, non-steroidal anti-inflammatory drugs, estrogen therapies, and some diabetes medications. Underlying medical conditions that can contribute to edema include congestive heart failure, kidney harm and kidney disease, disorders that touch the veins of the legs, and cirrhosis and other liver disorders.
Therapy for edema usually focuses on elimination of the cause. Activities that can reduce the effects of the condition include appropriate exercises to keep the blood and lymph flowing through the affected areas. Other therapies include elevation of the affected part to assistance drainage, massage and compression of the areas to move the fluid out of the tissues, and decreased salt intake to decrease sodium and water retentivity.
Affiliate Review
Your body is mostly water. Torso fluids are aqueous solutions with differing concentrations of materials, chosen solutes. An appropriate balance of water and solute concentrations must be maintained to ensure cellular functions. If the cytosol becomes also concentrated due to water loss, prison cell functions deteriorate. If the cytosol becomes too dilute due to water intake by cells, cell membranes tin can be damaged, and the jail cell can burst. Hydrostatic pressure is the force exerted by a fluid against a wall and causes move of fluid betwixt compartments. Fluid can also move betwixt compartments along an osmotic gradient. Agile send processes require ATP to motility some solutes against their concentration gradients between compartments. Passive transport of a molecule or ion depends on its power to pass easily through the membrane, too as the existence of a high to depression concentration gradient.
Cocky Check
Answer the question(s) below to see how well y'all understand the topics covered in the previous section.
Critical Thinking Questions
- Plasma contains more than sodium than chloride. How tin this be if individual ions of sodium and chloride exactly balance each other out, and plasma is electrically neutral?
- How is fluid moved from compartment to compartment?
Glossary
extracellular fluid (ECF): fluid exterior to cells; includes the interstitial fluid, blood plasma, and fluids constitute in other reservoirs in the torso
fluid compartment: fluid inside all cells of the torso constitutes a compartment system that is largely segregated from other systems
hydrostatic pressure: pressure exerted by a fluid against a wall, caused by its own weight or pumping forcefulness
interstitial fluid (IF): fluid in the modest spaces between cells not contained within blood vessels
intracellular fluid (ICF): fluid in the cytosol of cells
Is Sodium Extracellular Or Intracellular,
Source: https://courses.lumenlearning.com/suny-ap2/chapter/body-fluids-and-fluid-compartments-no-content/
Posted by: haltertrachattee1941.blogspot.com


0 Response to "Is Sodium Extracellular Or Intracellular"
Post a Comment